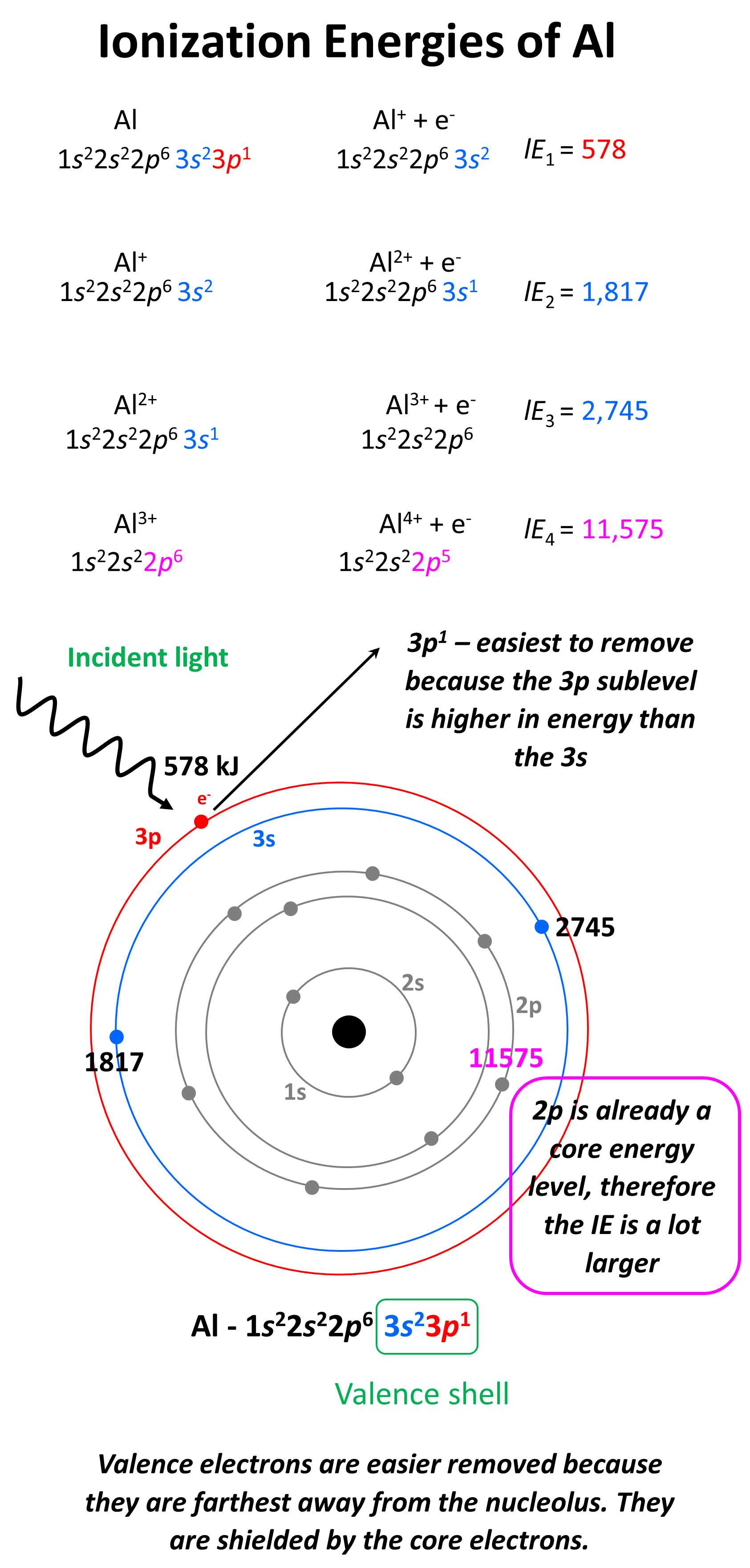
Does Chlorine Have A Higher Ionization Energy Than Aluminum . First ionization energy of chlorine is 12.9676 ev. 120 rows ionization energy chart of all the elements is given below. 1 ev/atom = 96.49 kj/mol. Notice that the general trend is upwards, but this is broken by. For example, magnesium has a higher ionization energy than aluminum. Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its valence. First ionization energy, second ionization energy as well as. An electron volt is the appropriate unit to describe. Values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. Magnesium has an electron configuration of [ne]3s2. Typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): The pattern of first ionisation energies across period 3.
from general.chemistrysteps.com
Values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. Typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): 1 ev/atom = 96.49 kj/mol. Magnesium has an electron configuration of [ne]3s2. Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its valence. Notice that the general trend is upwards, but this is broken by. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. An electron volt is the appropriate unit to describe. The pattern of first ionisation energies across period 3.
Ionization energy Chemistry Steps
Does Chlorine Have A Higher Ionization Energy Than Aluminum 1 ev/atom = 96.49 kj/mol. An electron volt is the appropriate unit to describe. 1 ev/atom = 96.49 kj/mol. Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its valence. Magnesium has an electron configuration of [ne]3s2. First ionization energy of chlorine is 12.9676 ev. For example, magnesium has a higher ionization energy than aluminum. 120 rows ionization energy chart of all the elements is given below. Notice that the general trend is upwards, but this is broken by. Values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. The pattern of first ionisation energies across period 3. Typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): First ionization energy, second ionization energy as well as.
From www.slideserve.com
PPT Learning Objectives •Define first ionisation energy and Does Chlorine Have A Higher Ionization Energy Than Aluminum First ionization energy of chlorine is 12.9676 ev. An electron volt is the appropriate unit to describe. For example, magnesium has a higher ionization energy than aluminum. Notice that the general trend is upwards, but this is broken by. 1 ev/atom = 96.49 kj/mol. Values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From www.creative-chemistry.org.uk
First ionisation energy across period 3 Creative Chemistry Does Chlorine Have A Higher Ionization Energy Than Aluminum The pattern of first ionisation energies across period 3. 1 ev/atom = 96.49 kj/mol. Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its valence. Typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): An electron volt is the appropriate unit to describe. Magnesium has an electron configuration of. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From chemwiki.ucdavis.edu
Periodic Properties of the Elements Chemwiki Does Chlorine Have A Higher Ionization Energy Than Aluminum 1 ev/atom = 96.49 kj/mol. First ionization energy, second ionization energy as well as. For example, magnesium has a higher ionization energy than aluminum. An electron volt is the appropriate unit to describe. The pattern of first ionisation energies across period 3. 120 rows ionization energy chart of all the elements is given below. Values for the ionization energies of. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From therealgroupichem.weebly.com
Ionization energy Group i Chemistry(iiiescuro) Does Chlorine Have A Higher Ionization Energy Than Aluminum The pattern of first ionisation energies across period 3. Values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its valence. First ionization energy of chlorine is 12.9676 ev. 1 ev/atom =. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From chem.libretexts.org
7.4 Ionization Energy Chemistry LibreTexts Does Chlorine Have A Higher Ionization Energy Than Aluminum An electron volt is the appropriate unit to describe. The pattern of first ionisation energies across period 3. For example, magnesium has a higher ionization energy than aluminum. First ionization energy of chlorine is 12.9676 ev. Magnesium has an electron configuration of [ne]3s2. First ionization energy, second ionization energy as well as. 1 ev/atom = 96.49 kj/mol. Notice that the. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From www.slideserve.com
PPT IB Chemistry ATOMIC THEORY PowerPoint Presentation, free download Does Chlorine Have A Higher Ionization Energy Than Aluminum 120 rows ionization energy chart of all the elements is given below. Magnesium has an electron configuration of [ne]3s2. Values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. The pattern of first ionisation energies across period 3. First ionization energy of chlorine is 12.9676 ev. Typical units for. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From neetlab.com
Ionization Enthalpy NEET Lab Does Chlorine Have A Higher Ionization Energy Than Aluminum The pattern of first ionisation energies across period 3. Typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): 1 ev/atom = 96.49 kj/mol. Notice that the general trend is upwards, but this is broken by. Magnesium has an electron configuration of [ne]3s2. First ionization energy of chlorine is 12.9676 ev. Values for the ionization energies of \(li\). Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From chemwiki.ucdavis.edu
5.6 Periodic Properties of the Elements Chemwiki Does Chlorine Have A Higher Ionization Energy Than Aluminum Notice that the general trend is upwards, but this is broken by. An electron volt is the appropriate unit to describe. 1 ev/atom = 96.49 kj/mol. First ionization energy, second ionization energy as well as. Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its valence. First ionization energy of chlorine is. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From courses.lumenlearning.com
Periodic Variations in Element Properties Chemistry I Does Chlorine Have A Higher Ionization Energy Than Aluminum Values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. Typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): Magnesium has an electron configuration of [ne]3s2. Notice that the general trend is upwards, but this is broken by. First ionization energy of chlorine is 12.9676 ev.. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From www.chemistrylearner.com
Periodic Trends Definition and Properties Does Chlorine Have A Higher Ionization Energy Than Aluminum First ionization energy, second ionization energy as well as. An electron volt is the appropriate unit to describe. 1 ev/atom = 96.49 kj/mol. Values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. First ionization energy of chlorine is 12.9676 ev. The pattern of first ionisation energies across period. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation Does Chlorine Have A Higher Ionization Energy Than Aluminum The pattern of first ionisation energies across period 3. An electron volt is the appropriate unit to describe. 1 ev/atom = 96.49 kj/mol. Magnesium has an electron configuration of [ne]3s2. First ionization energy, second ionization energy as well as. Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its valence. Values for. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From byjus.com
Does NaorMghave higher ionization energy? Does Chlorine Have A Higher Ionization Energy Than Aluminum Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its valence. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. 1 ev/atom = 96.49 kj/mol. Notice that the general trend is upwards, but this is broken by. An electron volt. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than Does Chlorine Have A Higher Ionization Energy Than Aluminum 1 ev/atom = 96.49 kj/mol. 120 rows ionization energy chart of all the elements is given below. An electron volt is the appropriate unit to describe. Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its valence. Values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Does Chlorine Have A Higher Ionization Energy Than Aluminum Magnesium has an electron configuration of [ne]3s2. An electron volt is the appropriate unit to describe. Values for the ionization energies of \(li\) and \(be\) listed in table \(\pageindex{1}\) show that successive ionization energies for an element. First ionization energy of chlorine is 12.9676 ev. For example, magnesium has a higher ionization energy than aluminum. Chlorine has a higher ionization. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Does Chlorine Have A Higher Ionization Energy Than Aluminum 1 ev/atom = 96.49 kj/mol. First ionization energy of chlorine is 12.9676 ev. Magnesium has an electron configuration of [ne]3s2. Typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): For example, magnesium has a higher ionization energy than aluminum. Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From sciencetrends.com
5 Fundamental Properties Of Nonmetals Science Trends Does Chlorine Have A Higher Ionization Energy Than Aluminum Notice that the general trend is upwards, but this is broken by. Typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): First ionization energy, second ionization energy as well as. 1 ev/atom = 96.49 kj/mol. Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its valence. An electron volt. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From byjus.com
11.What is the correct order of increasing second ionisation energy in Does Chlorine Have A Higher Ionization Energy Than Aluminum Typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): 1 ev/atom = 96.49 kj/mol. An electron volt is the appropriate unit to describe. For example, magnesium has a higher ionization energy than aluminum. First ionization energy, second ionization energy as well as. Magnesium has an electron configuration of [ne]3s2. Values for the ionization energies of \(li\) and. Does Chlorine Have A Higher Ionization Energy Than Aluminum.
From socratic.org
Which element has a higher 3rd ionization energy, Al or Mg? Why? Socratic Does Chlorine Have A Higher Ionization Energy Than Aluminum 1 ev/atom = 96.49 kj/mol. Typical units for ionization energies are kilojoules/mole (kj/mol) or electron volts (ev): First ionization energy, second ionization energy as well as. Chlorine has a higher ionization energy than aluminum because it has a greater effective nuclear charge on its valence. For example, magnesium has a higher ionization energy than aluminum. The pattern of first ionisation. Does Chlorine Have A Higher Ionization Energy Than Aluminum.